

About Us

OcyonBio provides dedicated autonomous manufacturing capacity with interconnected infrastructure & systems to support phased appropriate development for early development, pre-clinical, clinical, and commercial start.
Our mission is to make medicine affordable because it is the right thing to do. We differentiate ourselves by providing a 50% reduction in cost, a 700+ talent database to support hiring, and 200K sq. ft. of customizable space with cleanrooms from 200- 50,000 sq. ft.
With this intention, our Partnership Development Manufacturing Organization (PDMO) model aims to provide companies with their own space, so there is no need to build an expensive facility and transfer their IP to a CDMO. The customized lease space enables flexibility to our clients by allowing them to control schedules, select and manage resources, and introduce multiple products with a single capital expenditure.
Capabilities & Services

Process Development
Development/Optimization (PD/AD)
Technology Transfer (MS&T/AD)


Clinical & Commercial Manufacturing
Quality & Compliance
Comprehensive In-house
Quality Control Laboratories
Support of Facility and Operational Control
Supports the Complete Product Lifecycle
In-house manufacturing capabilities
Interconnected infrastructure & systems
Support phased appropriate development
(Early development, pre-clinical, clinical & commercial )

We provide Talent, Space, Speed & Reduce Cost of Critical Raw Materials to
Gene and Cell Therapy, Biologics, and Viral Vector clinical and Commercial Programs.
Reduce
Cost
OcyonBio combines our lowered cost vs.
the US mainland and the federally backed incentives offered by the Puerto Rico Government to reduce the overall cost by more than 50%.
The incentives apply to the construction, equipment, people, raw materials, and operational cost. The program aims to incentivize companies’ costly transition from R & D to commercialization.
The program offers 50% rebates based on annual spend for up to 15 years, or upon product commercialization, enabling organizations to achieve their goals by reducing costs by 50%. Our partnership model and value proposition make us unique compared to building your facility or contracting with a CDMO.
Addressing Industry Challenges
Critical
Raw Materials
Our campus has been designed to capture operational efficiencies by co-locating essential raw materials such as blood and viral vectors on the same campus. Our clients can take advantage of the blood processing unit, where our team can process blood and deliver the target cell to your manufacturing suite.
Additionally, our clients utilize our LENTI, RETRO, and AAV technology and facilities built in a dedicated location on our campus. The goal is to enable our partners to effectively use the complete supply chain for critical raw materials co-located on the same campus.

We push your vision forward
Talent
Scientific database with almost 700 scientists. 25,000 STEM Graduates, top 20 USA universities.
cGMP Spaces
Clean rooms 200-50,000 sq.ft.
From R & D to commercial mg.
Speed
Customizable within 60-180 days.
Ready to go clinical ISO 8 & 7
clean rooms.
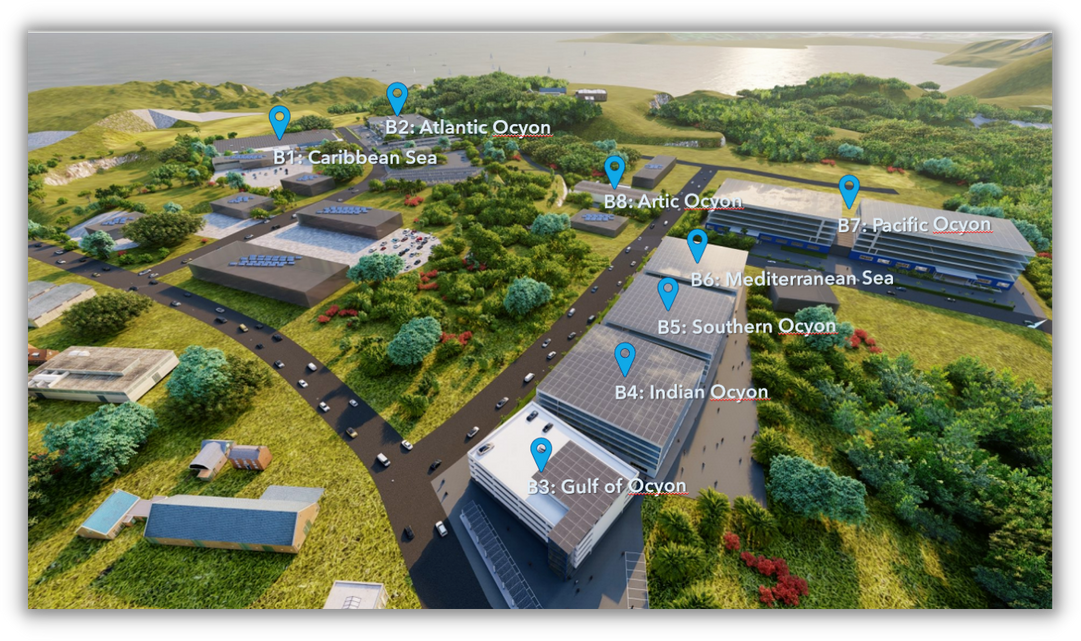
Our Campus
Experienced Scientific & Commercial Leadership
Robert Salcedo
Chief Executive Officer
Daniel Chang
Chief Financial Officer




25+ yrs in biotechnology & solid dose manufacturing.
Led facility development of Amgen Puerto Rico.






20+ yrs executive experience biopharmaceuticals &
biosimilars.
Led product development
for 30+ INDs.

Client Review

Get in touch
OcyonBio
Calle George Sanders
Bo. Camaceyes
Aguadilla, PR 00603
Email Address
connect with us

